Research Involving Human Tissue
🔍 Zoom in
Human Tissue Act (HTA) Notes to accompany flowchart
For similar flowcharts produced by the Human Tissue Authority relating to licensing, consent and ethical approval, see Appendices A and B of the → Human Tissue Authority's Code of Practice on Research.
Also, see the → UCL School of Life and Medical Sciences Human Tissue Act webpages and → UCL Biobanking.
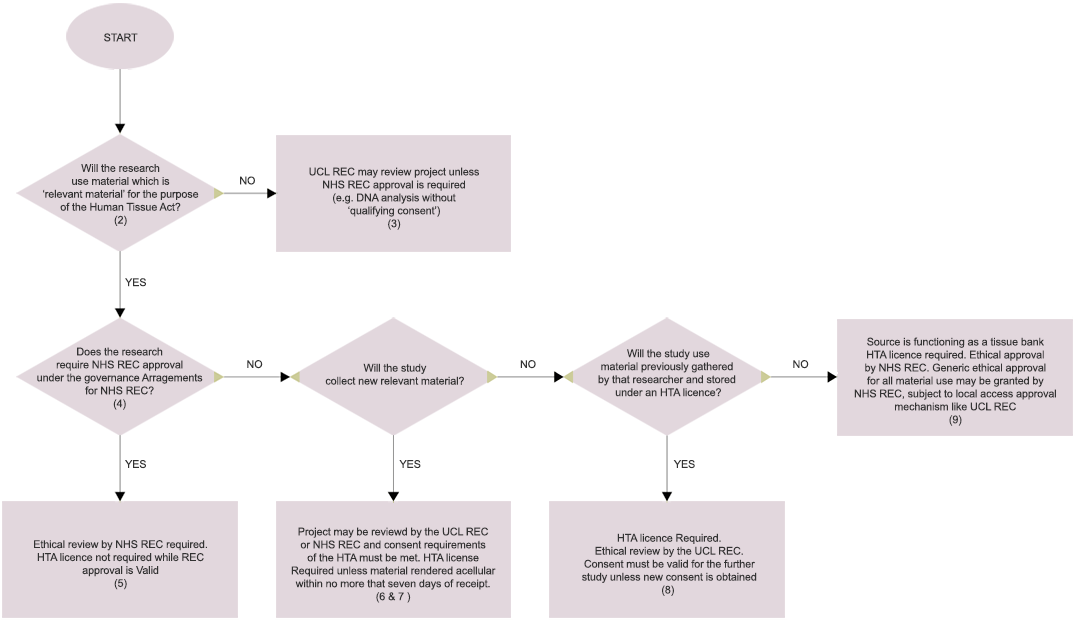
"Relevant material" is defined in s.53 of the HTA as material from a human body that consists of or includes cells. Hair and nails from the living are excluded from the definition, as are gametes and embryos outside the body. The Human Tissue Authority has produced a → Definition of Relevant Material and a Supplementary List of the most common types of materials.
NHS REC approval is not required for research involving acellular material (e.g. plasma, serum, DNA) unless the research involves:
- the collection of tissue samples from users of NHS or social care services for which the UK Health Departments are responsible (see note 4), in order to extract acellular material for the research;
- the collection of information from users of these services;
- use of previously collected information from which users of these services could be identified by the researchers; or
- the analysis of DNA in material from the living, where consent for research is not in place from the person whose body manufactured the DNA.
See the → National Research Ethics Service's (NRES's) algorithm for determining whether research requires NHS REC approval through the → IRAS application process.
While DNA is not "relevant material" for the purposes of the HTA, DNA analysis without "qualifying consent" is an offence under the HTA subject to certain exceptions. Research involving DNA analysis without consent requires the approval of an NHS REC and participants must be non-identifiable to the researcher: see → HTA Code of Practice 9 – Research.
Where DNA analysis is conducted with consent, the UCL REC must ensure that the requirements of the Act for 'qualifying consent' are met.
Broadly speaking, NHS REC approval is required for research projects involving:
- participants identified due to their past or present use of NHS or social care services for which the UK Health Departments are responsible;
- participants identified because of their status as relatives or carers of users of these services;
- the collection of tissue or information from users of these services; and
- use of previously collected tissue or information, from which past or present users of these services could be identified from the tissue/information on its own or through combination with other tissue/information.
NHS REC approval is no longer required for research involving NHS staff or NHS premises unless it is also required by one of the above criteria. NHS REC approval is not required for research involving previously collected, non-identifiable human tissue samples (e.g. from NHS patients), unless one of the following conditions applies: (1) consent has not been given or the research is outside the scope of the consent; (2) the material is not held on premises covered by an HTA licence; (3) the project also involves the use of new samples gathered from the living or the deceased; or (4) the research also involves the use of identifiable information held with the samples. See → Governance Arrangements for NHS Research Ethics Committees.
Licensing requirements: Under the Human Tissue Authority's legislation, no licence is required for human tissue which is held for a research project which has been approved by an NHS REC. However, a licence is necessary once NHS REC approval has expired unless new approval is obtained.
A list of → Human Tissue Authority licensed establishments at UCL.
A study collecting new relevant material must hold the material under an Human Tissue Authority licence unless NHS REC approval has been obtained or the material will be rendered acellular within no more than seven days of receipt. Where ethical review is conducted by the UCL Research Ethics Committee, the committee must ensure that consent is obtained in accordance with the requirements of the HTA, as the exception allowing research without consent (which requires NHS REC approval) will not apply.
"Relevant material" excludes material which has been processed in such a way as to render it acellular: e.g. serum and plasma which contains no platelets or other blood cells. The Human Tissue Authority's guidance is that a licence will be required where human tissue is stored prior to processing it to render it acellular. However, no licence is required where the processing takes place within "a matter of hours or days and certainly no longer than a week" of receipt. Seven days should be seen as an absolute maximum (e.g. to allow for weekends or Bank Holidays) and processing should normally take place well before that. The Authority regards this as analogous to the temporary storage of tissue prior to transportation, which is subject to an exception in the Act.
Future research by a researcher using material previously gathered by them and stored under an Human Tissue Authority licence will require NHS REC approval if it falls within the Governance Arrangements for NHS RECs. Otherwise, ethical review may be conducted by the UCL Research Ethics Committee, which must ensure that the consent requirements of the HTA are met. The study must be consistent with the consent given in the initial project unless further consent is obtained.
Tissue banks must hold an Human Tissue Authority licence and can apply for generic NHS REC approval. Where the tissue is obtained from a tissue bank with generic approval, no licence is required for the study and the research will be subject to the conditions imposed by the NHS REC approval. The UCL Research Ethics Committee may function as a local access committee to review applications to ensure that the proposed study meets the conditions stipulated by the NHS REC approval. Once the study has ended, the material must either be disposed of, or transferred back to the tissue bank, or held under an Human Tissue Authority licence or under a new NHS REC approval.
Where material is obtained from a tissue bank which does not have generic NHS REC approval, the researcher must hold it under an Human Tissue Authority licence and obtain NHS REC approval.
May 2012
 Close
Close

